Disclaimer: links to web sites are ever-changing.
It turns out to be a Sisyphus task to keep them updated all the time.
Therefore, either try a different "spelling" of the hyperlink, look for
it on google.com and/or let me know about an outdated link by writing an
e-mail to aveh@wncc.net .
Light
and Matter
The
electromagnetic (EM-) spectrum
(link doesn't work)
g rays -
-
-
-
-
- radio
(check your astronomy book and/or look below)
All of the above are of the same nature: they are part of the electromagnetic
spectrum (Snow's Universe is temporarily offline).
Differences occur only because they have different frequencies, wavelengths
and energies. Differences for us occur, because we use or perceive them
for/as different purposes/hazards. Stars radiate all kinds of EM
waves, how much of each kind depends on the star's surface temperature
(Planck's radiation law).
A very pretty diagram can be found at Microsoft's
Encarta.
Frequency: ___ creases from left to right
Wavelength: ___ creases from left to right;
Speed of light = Frequency times Wavelength
Energy: ___ creases
from left to right; Energy = Planck's constant times
Frequency
This means that frequency, wavelength and energy are interrelated. Whenever
the talk is about radiation, the terms frequency, wavelength and energy
could be used interchangeably. It's customary though to designate
low energy EM waves with frequency (e.g. the MHz for FM radio), medium
energy EM waves with wavelength (e.g. nanometers or Angstrom for visible
light), and high energy EM waves with energy (electronvolts for x-rays
and g-rays).
An interesting feature of EM radiation is that it can appear as a wave
or as a particle, named the photon. We speak of EM radiation as photons
when they're send out during nuclear fusion of atomic nuclei and when they're
absorbed or emitted by atoms. EM radiation is eventually radiated from
a star. After traveling through space finally reaches our telescopes,
it is refracted by lenses or reflected by mirrors, both of which are indicative
of the wave properties, so we speak of waves.
Due to its composition of gases, the Earth's atmosphere is opaque to
some frequencies, and transparent to others. Of course much visible
and infrared reaches the Earth's surface and, luckily, most UV and x-rays
are absorbed by the atmosphere (e.g. ozone).
During the lectures, on websites and in textbooks, you'll notice that
different words are used to describe the same thing. Don't be too
confused by that. Instead of EM (Electromagnetic) the term "light"
is often used, meaning all radiation from radio to gamma, what we perceive
as light then, white, red to purple, is then referred to as "visible light".
EM (or "light") can be described as a photon or a wave, as radiation or
spectrum. Frequency, wavelength and energy are used exchangeable
courtesy the above mentioned preferences. Makes sense?

Radioactive sources producing a,
b, g rays (alpha are high-energy Helium nuclei, beta
are high-energy electrons, gamma are high-energy EM radiation). |

An x-ray tube: electrons are burnt off the cathode on
the left, slam into a tilted metal target in the middle, whereupon they
lose their energy and produce x-rays which leave the tube at a right
angle. |

A "black light" sends out ultraviolet as well
as visible light from the adjacent purple. The high energy UV raises
an electron to a quite higher orbit, from where it cascades down, thereby
emitting photons of lesser energy, i.e. visible light of various colors.
These are the fluorescent minerals in the photos).
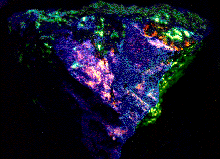
Clinohedrite, Hardystonite, Willemite, Franklin, NJ,
3 x 4" (SW); w/o permission from Ken's
Fluorsecent Minerals.
|
|
|

The familiar visible light froma a halogen light
bulb. It's spectrum is produced by a diffraction grating (see my
lab F5 Spectra). The human
eye recognizes those frequencies as colors. |

The "1985
Astronomy Song"
The radio is assembled from a Radio Shack kit.
Its antenna received radiowaves in the AM short wave band, its circuit
filters out the (still electronic) sound frequencies and the loudspeaker
converts them to audible sound. (You don't listen to the EM radiowaves
themeselves, you listen to the sound waves carried along by the EM radioaves.) |

The klystron on the left (the black cylinder on top)
contains an antenna producing microwaves. The receiver on
the right shows the intensity.
This experiment also shows an important property of EM
waves: they can be polarized, so that a grid parallel to the electric field
absorbs them (top) while a perpendicular lets them through. |

This candle produces heat. Actually, most of the
apparatus shown here produces heat and would show up brightly in an infrared
picture: the radio's amd loudspeaker's batteries, the microwave's klystron,
the halogen lamp, the black light, the x-ray tube, the radioactive sources. |
Temperature scales
(at
King's University)
Kelvin (British) - Celsius (Swedish) - Fahrenheit (German)
Kelvin is used in science because there are no negative temperatures
in Kelvin (which makes calculations easier). The Kelvin defines Absolute
Zero (nothing can be colder than that).
Temperature is a measure of the motion of atoms, molecules, and free
electrons.

Low Temperature.
High Temperature.
Motion ceases at Absolute Zero. That's why the Kelvin is so handy:
0 K means no motion of molecules.
Temperature, and therefore the amount of motion, is insofar important
as atoms collide more often at higher temperatures (how many collisions
depends on density as well) and thereby transfer energy from one atom to
another. Collisions can ionize atoms (remove an electron) or merely
"excite" atoms (make an electron jump up) which results in the emission
of an EM photon when the electron jumps back down. And as we know,
EM radiation is the most important stuff for astronomers.
Commonly used temperatures
| Absolute Zero |
0 K |
- 460 F |
| Cosmic background radiation |
3 K |
- 455 F |
| Room temperature |
295 K |
80 F |
| Interstellar space |
100 to 10,000 K |
- 250 to 20,000 F |
| Surface temperatures of stars |
3,000 to 50,000 K |
5,000 to 100,000 F |
| Core temperatures of stars |
10 to 500 Million K |
20 to 1,000 Million F |
Black Body
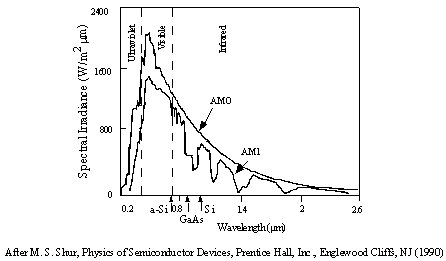 A
hot body (e.g. a star's surface) emits all frequencies of the EM-spectrum,
each with an intensity according to a black
body / Planck curve.
A
hot body (e.g. a star's surface) emits all frequencies of the EM-spectrum,
each with an intensity according to a black
body / Planck curve.
Wien's
law tells us where the peak
frequency (Snow's Universe is temporarily offline)
is.
The Stefan-Boltzmann
law tells us how much energy is radiated.
From this follows that both laws depend on temperature, i.e. if the
peak of a star's black body curve is known, its surface temperature can
be determined.
The
Atom
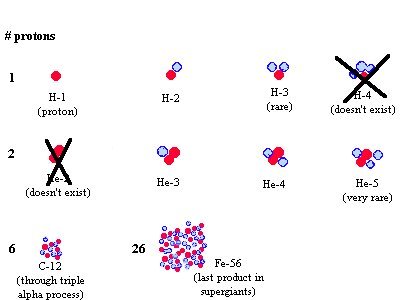
Nucleus: PROTONS (positive charge) - number of p
is responsible for which element is at hand
NEUTRONS (neutral), different number of n makes
different "isotopes" of the same element (same number of p )
Shell: ELECTRON (negative), same number of e
as p if the atom is neutral.
Check a table of the periodic system (see Johnson
Memorial Elementary School) or one in your textbook. The
following diagram shows only the nuclei of some elements but omits their
shells (electrons). It is therefore more useful for the discussion
about nuclear fusion in my Sun lecture.
An electron "jumps" from a higher to a lower shell: a PHOTON (i.e. a
light particle of certain frequency of EM-radiation) is emitted.
A photon is absorbed by an electron: the electron "jumps" from a _________
to a _________ shell.
Most atoms in stellar atmospheres are either neutral (#e = #p) or singly
ionized (#e = #p - 1), some atoms have more than one electron removed (#e
< #p - 1). Differently ionized atoms of the same element make
for different spectral lines, e.g. HeI (2 electrons) and HeII (1 electron).
Spectral lines
Kirchhoff's
laws: (at ZEBU)
(and
at Strobel's lightspeed)
-
Continuous spectrum - e.g. a light bulb, black body (a star's surface/photosphere).

-
Emission spectrum - a hot gas emits a certain spectrum (its emitted frequencies
are like a fingerprint to identify the gas).
 Sodium.
Sodium.
 Hydrogen.
Hydrogen.
-
Absorption spectrum - a gas (e.g. a star's
atmosphere (Snow's Universe is temporarily offline))
absorbs certain frequencies - the same frequencies that it would emit,
if it were hot, i.e. once again the frequencies are like a fingerprint(Snow's
Universe is temporarily offline). Below is our Sun's spectrum.
(c)
Mees Solar Observatory, Hawai'i.

Do you see some Sodium and Hydrogen lines?
-> Lab F5 Spectra of gases
Observing spectral lines reveals:
(Snow's Universe is temporarily offline) Chemical
Composition of star's atmosphere, temperature
of star's surface, rotation
of star (Doppler effect), radial motion
(Doppler) and binary
stars, electric/magnetic
fields, ...
As an example:
Doppler
Effect
(and
at Snow's Universe) (Snow's Universe is temporarily
offline
When a train/police car approaches, the pitch of its whistle/horn becomes
___________, when it recedes, its pitch becomes ___________.
Light is a wave just as sound is, so the same effect happens to
light:
It's a ______ shift, when approaching, and a _______ shift, when receding.
The light doesn't become blue/red, but it's shifted towards the blue/red
side of the spectrum. (Example from CLEA's Stellar Spectra
Lab)
Doppler shift
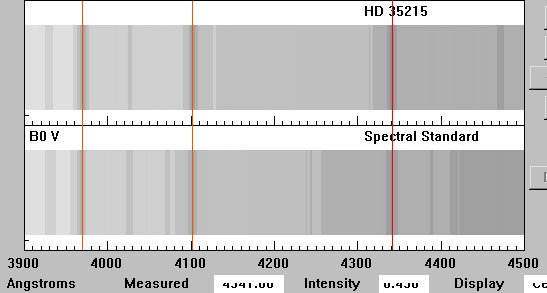
Wavelengths l are in Angstrom. This
CLEA software produces only the purple part of the spectrum since that
information is sufficient for the analysis we're doing. Deep purple
is at left (hardly noticeable at all to the human eye), deep blue on the
right.
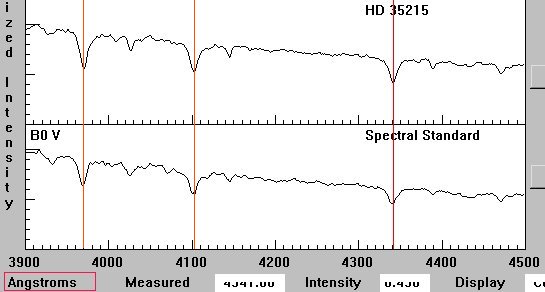
This intensity graph shows clearly the spectral lines of HD 35215 are
shifted towards the red when comparing them to a standard B0 V Main Sequence
star.
Is this star receding or approaching?
Doppler shift of spectral standard versus HD 35215.
|
standard l |
measured l |
Dl |
v = c (Dl) / (l) |
| Ca II (H-line) |
3970.4 |
3969.0 |
|
|
| H I (H d) |
4102.5 |
4101.2 |
|
|
| H I (H g) |
4341.3 |
4340.4 |
|
|
Determine how fast this star moves by using the formula for the Doppler
shift: v = c (Dl) / (l)
with c = 186,000 miles/sec.










 A
hot body (e.g. a star's surface) emits all frequencies of the EM-spectrum,
each with an intensity according to a
A
hot body (e.g. a star's surface) emits all frequencies of the EM-spectrum,
each with an intensity according to a 

